Ferla MP, Patrick WM. Bacterial methionine biosynthesis. Microbiology. 2014 Aug;160(Pt 8):1571-84.
PMID: 24939187, doi: 10.1099/mic.0.077826-0 and pdf.
PMID: 24939187, doi: 10.1099/mic.0.077826-0 and pdf.
The MetCombo
It is my opinion that a bifunctional enzyme that catalyses both the MetC and the MetB reaction is impossible. I have come to call this hypothetical enzyme, the MetCombo. So the data at hand are:- MetC and MetB are close homologues and it is really hard to tell them apart in a phylogram —with the bold assumption that the uncharacterised genes are what have been guess.
- Both KEGG and EcoCyc take the close homology to mean that bifunctional enzyme is present in several organisms —basically all those with MetB, which is a lot as you know from the met biosynthesis paper
- They are in the same pathway
- Nobody has ever seen a metCombo
- Papers that try to evolve MetC ↔ MetB are not realy successful
- Personal results: E. coli metC cannot rescue metB
- Personal results: Thermotoga maritima "metB" is actually a metC and it has no in vivo or in vivo MetB activity(check out my thesis)
- Catalytically a metC and metB in a single active site would be a disaster.
Catalytic profligacy
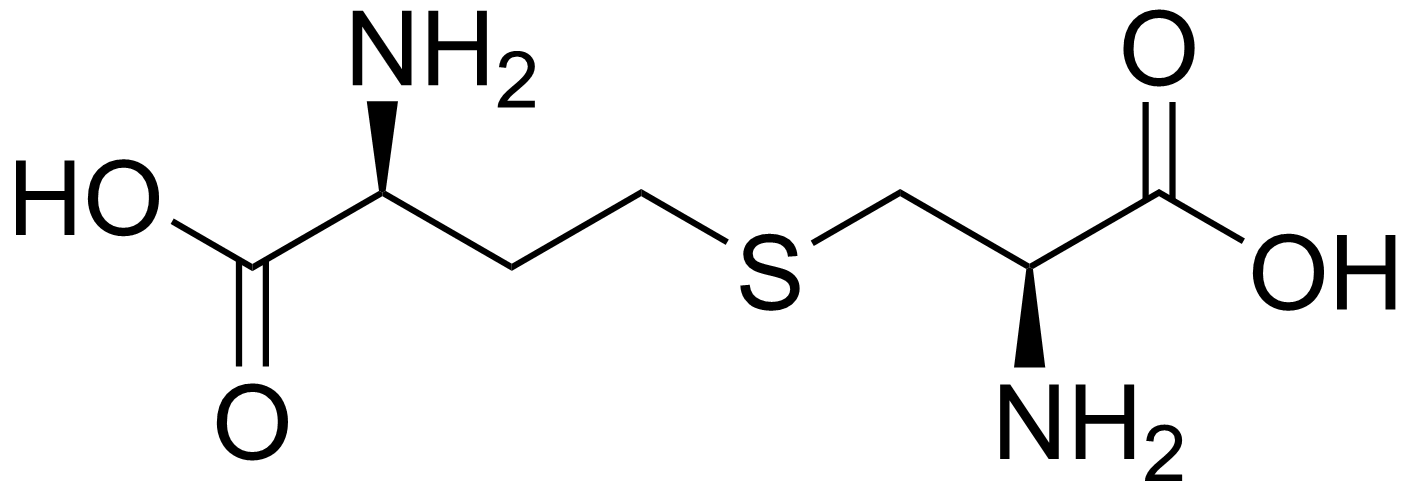
Cystathionine is a cysteine/alanine and a homocysteine/homoalanine joined together with a thioether. It has a short side (S is on the β) and a long side (S on the γ).MetC is cysthationine β-lyase, it eliminates cystathionine at the thioether bond. On the shorter side (β).
MetB is cystathionine synthase it eliminates O-acetyl-homoserine at the ester bond and then attacks it with cysteine's thiol making cystathionine.
The two PLP enzymes hold cystathionine at some point but in radically different ways, one on the β side (MetC), the other on the γ (MetB).
Taking a step back, we have two types of cystathionine lyase and what controls the specificity between a β-lyase and a γ-lyase is not known —there have been a few papers looking into making MetC into a MetB and viceverse, but unfortunately nothing tackling this simpler issue. Cystathionine looks nearly identical from both sides: the sulfur bridge is hard to tell apart from a methyl group as there is only a slight size and charge difference. Methionine can be substituted with norleucine in protein with only minimal effect. Therefore it is intriguing how the enzymes bind it tightly in a specific way. My theory is that sulfur-π interactions may be involved as there are several tyrosines in the active site of MetC. Additionally, a β-elimination might be easier than a γ-elimination, therefore it is shame that there is a decent amount of data of the lack of γ-elimination activity in the β-lyase, but not viceversa. Therefore it would seem more likely to have a powerful bifunctional β- γ- cystathionine lyase than have retrained one that is strongly specific. However, this bifunctional enzyme is not an evolutionary a good idea due to the number of round trips it would do. Specifically, cysteine or homocysteine would go into making cystathionine, which the uncommitted lyase would either correctly transform or return a starting substrate —at the cost of ATP. The reason for this fascinating parenthesis is to conclude that cystathionine synthase/lyase combo that could do both, would be equally as bad of an idea —it would work due to flux from excess substrate to product in demand, but it is just extremely inefficient.
Conflicting results
Some methionine gene rescue experiments go in different ways that expected and there occasionally are concentration dependent oddities. My opinion is that this is due toone of the following:- It is dominating the threonine branch point and there is not enough threonine being produced.
- It is depleting all the cysteine or homocysteine